About
ACTION3 study
The ACTION3 Study will examine if the investigational medicine, DMX-200 (repagermanium), reduces the amount of proteinuria (protein in your urine) and slows the decline of kidney function, when taken in addition to a medicine called an angiotensin II receptor blocker (ARB). An ARB is a very commonly used medicine in FSGS. ACTION3 will also investigate what side effects may occur.
The ACTION3 study will compare the investigational medication with a placebo. Both the investigational medication and placebo will be given as capsules that will need to be taken twice daily by mouth. You will be randomly assigned to either study drug or the placebo group. You have an equal chance (50%) to being in either group. Neither you nor the study team will know if you are receiving the study drug or the placebo.
This is a global study involving approximately 300 patients.
If the study is right for you and you choose to participate, all study-related care and study medication will be provided at no cost to you during your participation.

What is FSGS?
About Focal Segmental Glomerulosclerosis (FSGS)
FSGS is a progressive kidney disease for which there are no formally approved treatments. When you have FSGS, the filters (glomeruli) of your kidneys become inflamed and are damaged by scarring. This makes the filters “leaky” and allows protein from your blood to collect in your urine (proteinuria).
For patients with FSGS, the kidneys’ ability to purify (clean) the blood is impaired. This can eventually lead to kidney failure that may eventually requires dialysis or a kidney transplant.
Find out more about FSGS here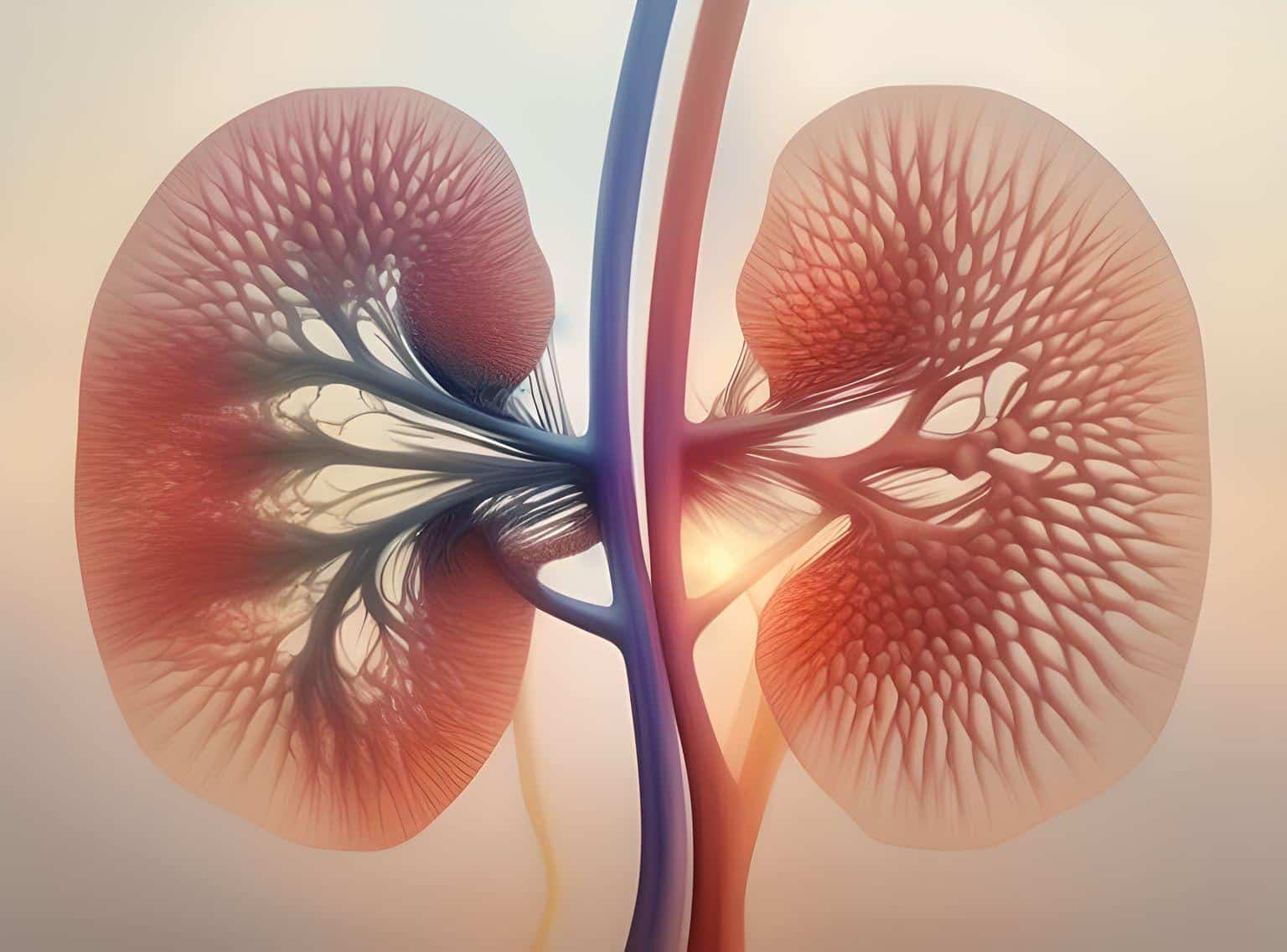
Study Design
Test and assessments
What tests and assessments can I expect?
The following tests and assessments will occur at some or all of your study visits.
Study Locations
If you are interested in participating, please use to the map below to contact your closest participating site and request for more information on the ACTION3 Phase 3 FSGS study in kidney disease, sponsored by Dimerix.
Alternatively please contact your treating physician and request they contact Dimerix at [email protected].
For confidentiality purposes, we request patients do not contact Dimerix directly.